Catalyzing Commercialization Sugar could some day be used to power smartphones, tablets, and other electronic devices thanks to a recent breakthrough by Blacksburg, VA-based Cell-Free BioInnovations, Inc. It might seem strange to use an ingredient found in cupcakes and cookies as an energy source, but it’s not, as most living cells break down sugar to produce energy. And, interestingly, the energy density of sugar is significantly higher than that of current lithium-ion batteries.
Working under a Small Business Innovation Research (SBIR) grant from the National Science Foundation, a research team led by Y-H Percival Zhang, Chief Science Officer of Cell- Free BioInnovations and an associate professor of biological systems engineering at Virginia Tech, has successfully demonstrated the concept of a sugar biobattery that can completely convert the chemical energy in sugar substrates into electricity.
As reported in the January 2014 issue of Nature Communications, this breakthrough in sugar-powered biobattery can achieve an energy-storage density of about 596 A-h/kg — an order of magnitude higher than the 42 A-h/kg energy density of a typical lithium-ion battery.
A sugar biobattery with such a high energy density could last at least ten times longer than existing lithium-ion batteries of the same weight, drastically reducing how often users need to recharge their electronic devices. This nature-inspired biobattery is a type of enzymatic fuel cell (EFC)— an electrobiochemical device that converts chemical energy from fuels such as starch and glycogen into electricity.
While EFCs operate under the same general principles as traditional fuel cells, they use enzymes instead of noble metal catalysts to oxidize the fuel. Enzymes allow for the use of more-complex fuels (e.g. glucose), and these more-complex fuels are what give EFCs their superior energy density. For example, the complex sugar hexose can release 24 electrons per glucose molecule during oxidation, whereas hydrogen (a fuel used in traditional fuel cells) releases only two electrons. Until now, however, EFCs have been limited by incomplete oxidation, releasing just two to four electrons per glucose molecule.
“We are not the first who proposed using sugar as the fuel in the biobattery,” says Zhiguang Zhu, a senior scientist at Cell-Free BioInnovations. “However, we are the first to demonstrate the complete oxidation of the sugar in the biobattery, enabling our technology to have a near-theoretical energy conversion yield that no one has ever reported.”
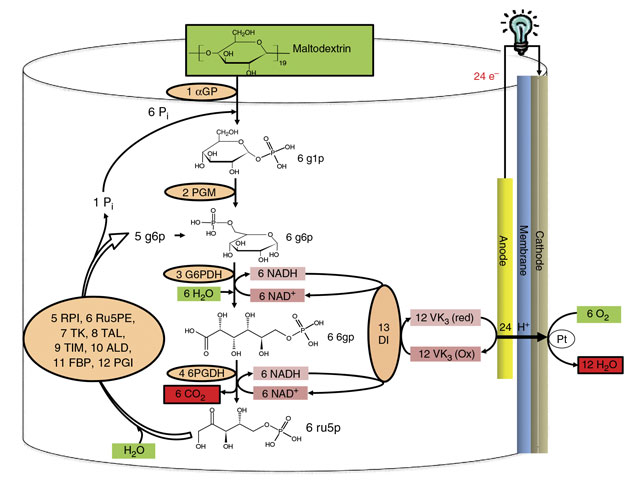
Zhang and his team constructed a synthetic catabolic pathway (a series of metabolic reactions that break down complex organic molecules) containing 13 enzymes to completely oxidize the glucose units of maltodextrin, yielding nearly 24 electrons per glucose molecule.
We put specific thermostable enzymes into one vessel to constitute a synthetic enzymatic pathway that can perform a cascade of biological reactions the sugar, converting it into carbon dioxide, Zhang says. Unlike natural catabolic pathways for the oxidation of glucose in cells, the designed synthetic pathway does not require costly and unstable cofactors, such as adenosine triphosphate (ATP), coenzyme A, or a labile cellular membrane. The researchers used two redox enzymes that generate reduced nicotinamide adenine dinucleotide (NADH) from sugar metabolites. NADH, a reducing agent involved in redox reactions, is a natural electron mediator that carries electrons from one molecule to another. They also used ten other enzymes responsible for sustaining metabolic cycles and an additional enzyme that transfers electrons from NADH to the electrode.
This new synthetic pathway enables the biobattery to extract the theoretical number of electrons per glucose unit and thereby use all the chemical energy in the sugar. This, the team reports, represents a significant breakthrough.
In addition to its superior energy density, the sugar biobattery is also less costly than the Li-ion battery, refillable, environmentally friendly, and nonflammable. While researchers continue to work on extending the lifetime, increasing the power density, and reducing the cost of electrode materials for such a battery, they hope that the rapidly growing appetite for powering portable electronic devices could well be met with this energy dense sugar biobattery in the future. For the Silo, Zhiguang Zhu, chief scientist at”The Sweet Battery Project”.
This technology was funded through the America’s NSF Small Business Innovation Research Program.